Lynch Syndrome – Endometrial Cancer Pathway Supporting Documents
The supporting documents have been developed to further help you to follow the Lynch syndrome Quality Improvement Programme pathway. Some are specific documents to help you follow the pathway, some to support the MDT members to follow the pathway, and some others to support individual clinicians to request the necessary tests, support them with genetic counselling, requesting genetic testing, and giving the results. Here you can also find the guidelines to help you make the appropriate recommendations to your patients and their family, and the link to the patient information website and other patient resources.
Lynch syndrome quality improvement project flowcharts
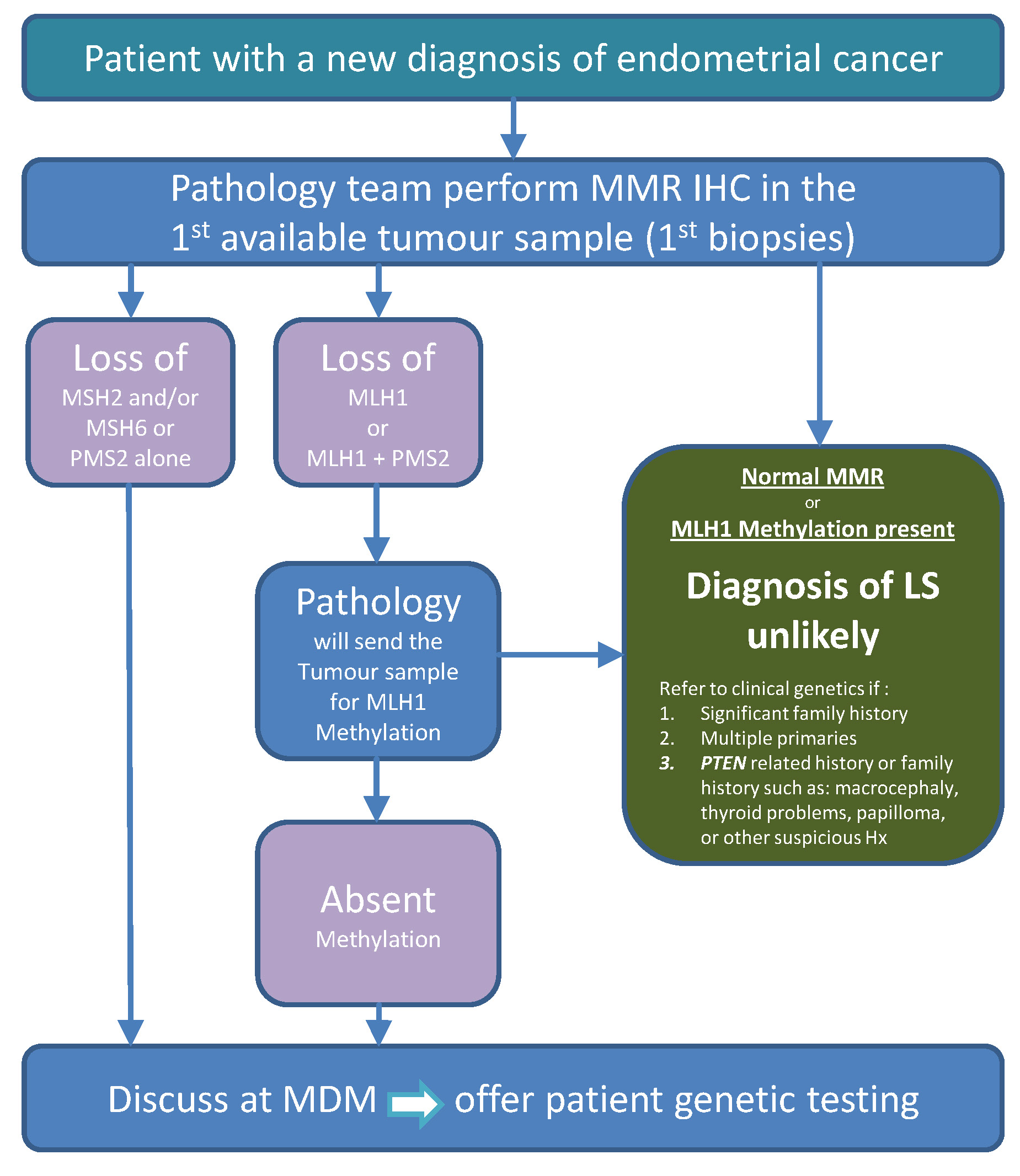
Tumour testing: Identifying patients eligible for genetic testing
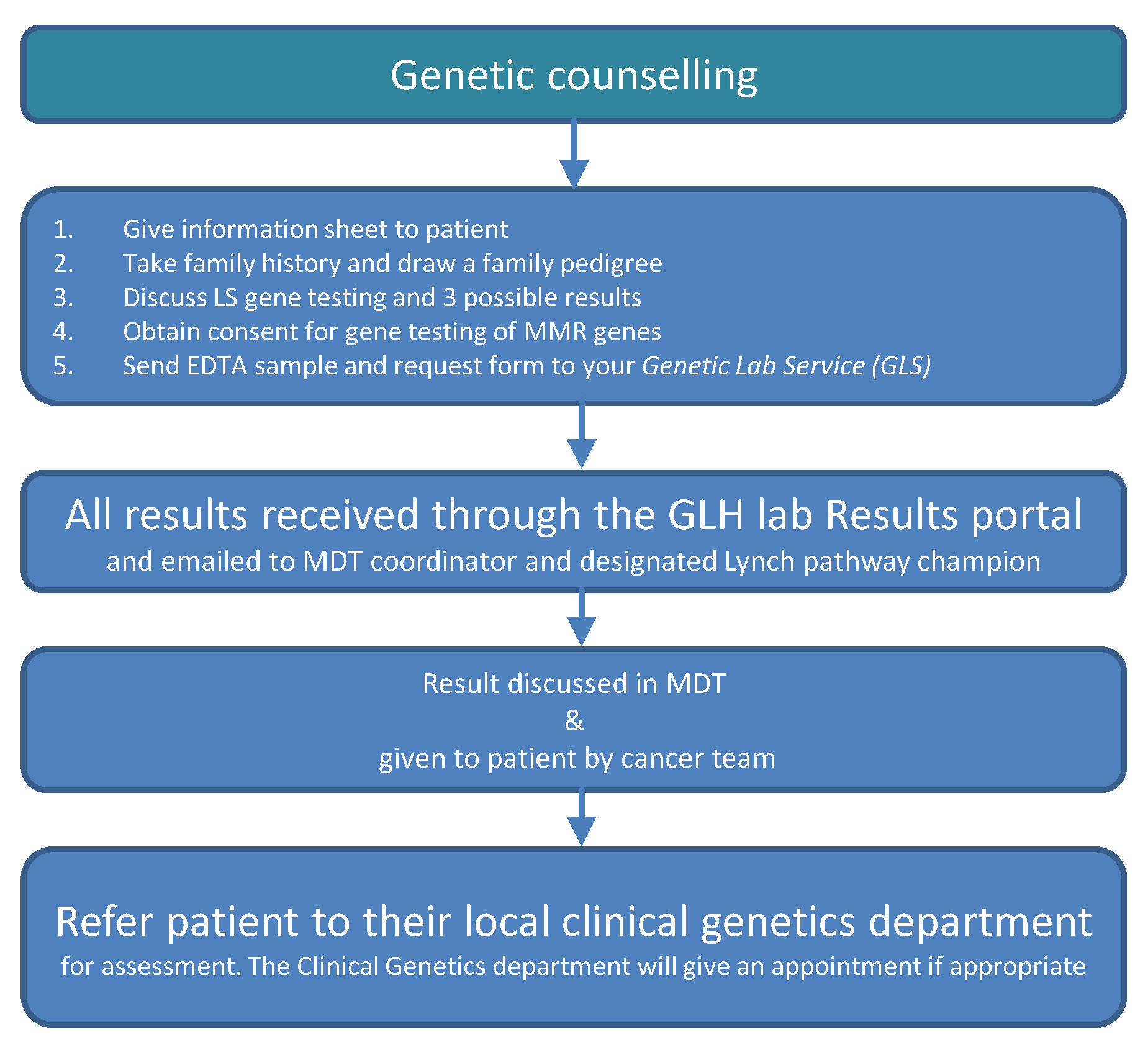
Genetic counselling: Consenting patients for genetic testing and giving results
Lynch Syndrome QIP guidance for the oncology MDM in RM Partners allied Trusts
This SOP aims to guide members of oncology MDMs in participating hospitals to follow the Lynch syndrome QIP early diagnostic pathway. It aims to explain the steps that MDMs need to follow, and to clarify the MDM’s responsibility with regards to what tests need to be requested, discussed, and documented in the MDM outcome.
Lynch syndrome Standard Operating Procedure (December 2021)
Discussing results in our regional genetics MDT meeting
Results and surveillance recommendations may be discussed in regional specialised cancer genetics MDT meeting, e.g. St Mark’s virtual (Microsoft Teams) Hereditary Cancer MDM Tuesdays at 9am. If you would like to attend this meeting or present a case, you can email LNWH-tr.SMCFIC@nhs.net for the Microsoft Teams access details.
If you would like to present a case, please email this proforma to LNWH-tr.SMCFIC@nhs.net
Virtual Hereditary Endometrial Cancer MDT proforma
Example results letter
This document has been created to give an idea of what to write in the genetic results letter. It is not intended to use as a template, but rather to give an idea on how a genetic result letter could be written
Lynch syndrome guidelines
- Guidelines for the management of hereditary colorectal cancer
- The Manchester recommendations for the management of gynaecological cancers in Lynch syndrome
- NHS England Implementing Lynch syndrome testing and surveillance pathways Version 1.2 (updated 12 September 2023)
Request and consent forms
In the right hand column you can find the request forms needed to perform further testing on the tumour sample (if there is loss of MLH1 or MLH1 + PMS2); or to consent and request genetic testing during your genetic counselling consultation. Find the following forms:
- Consent form for genetic testing for Lynch syndrome (all hospitals should use this form)
- Request form: Tumour testing for MLH1 promoter hypermethylation (use your designated molecular laboratory form)
- Request form for genetic testing for Lynch syndrome: EDTA blood bottle (use your hospital’s designated molecular laboratory form)
Please note, each hospital should know which molecular laboratory they are linked with.
Lynch syndrome patient resources
- Patient leaflet: Information for Patients with Endometrial Cancer July 2021: Your genetics appointment and testing for Lynch syndrome . This document is mentioned in the training. Ideally, it should be given to eligible patients prior their appointment for genetic counselling for Lynch syndrome.
- Patient specific web pages: If your patient has further questions about Lynch syndrome or genetic testing you can direct them to the dedicated patient specific information web page.
- Patient information resources including leaflets are available here.