Lynch Syndrome Quality Improvement Project
The Lynch Syndrome (LS) quality improvement project has been developed to ensure an effective and fast diagnostic pathway from diagnosis of colorectal cancer to diagnosis of LS. The aims are to:
- Meet the NHS Long Term Plan ambition to diagnose cancer at an early stage. If all people with colorectal cancer and their family members were tested for LS and enrolled into appropriate surveillance pathways, by 2028 we would expect up to 380 more colorectal cancers to be diagnosed early. That’s up to a 0.9% point increase against the Long Term Plan ambition to diagnose 75% of cancers early by 2028.
- Increase the identification and diagnosis of LS. This is a top priority, as it is estimated that we only know about 5% of the LS cases. It is estimated that this increased identification and diagnosis of LS could save over 300 lives annually in England.
- Improve cancer prevention through identification of individuals at risk by genetic testing of unaffected family members through cascade testing. Cost effective treatments and services are available to help people with LS manage and reduce their risk.
- Implementing stratified medicine, including optimal treatment selection and surveillance packages, to prevent cancer recurrence
With this strategy we will maximise opportunities to save the lives of patients with LS, and those with sporadic Microsatellite Instability (MSI) tumours who have a diagnosis of colorectal cancer, and the lives of their asymptomatic relatives. This can ensure minimal variability in patient care, appropriate surveillance and implementation of other broader prevention and treatment strategies.
Background
The NHS Long Term Plan sets an ambition that by 2028, 75% of cancers will be diagnosed at an early stage. One of the ways this will be reached is through targeted screening and personalised surveillance of those most at risk of developing cancer, such as those with LS.
The annual incidence of colorectal cancer (CRC) in the UK is approximately 35,000, with an individual lifetime risk of 5.5%. Up to 35% of all cases of CRC in the UK arise due to heritable risk factors, and of these, 2-3% are caused by LS. Thus, LS is thought to account for approximately 1,200 cases of CRC annually in the UK, and a similar number of extracolonic cancers, especially endometrial cancer in women. In addition a further 15% of CRCs manifest tumour features similar to LS (somatic MMR deficient tumours, i.e. due to non-inherited causes).
LS is the most common form of hereditary CRC. An estimated 175,000 people have LS in the UK, but fewer than 5% of individuals know they have the condition (Bowel Cancer UK).
For this reason, in February 2017 NICE recommended universal testing for LS for all newly diagnosed cases of CRC (DG27). It is thought that 300 lives may be saved annually with full implementation of this guideline, as diagnosis in CRC patients and their relatives provides many opportunities for personalised care and prevention strategies, including surveillance colonoscopy, aspirin as prophylaxis, risk reducing gynaecological surgery, and adaptive oncological therapies in CRC patients.
A diagnosis of deficient MMR (dMMR) can affect cancer treatment options, with certain tumours being more responsive to particular chemotherapy agents. People with LS are also responsive to new immunotherapy drugs. It is therefore important that the initial tumour test (IHC or MSI) is done in time to inform treatment options.
A multidisciplinary expert letter published in the BMJ in 2017 recommended a national and regional strategy for the management of these patients with the following specific goals:
- The development of a national LS registry
- A quality assured surveillance programme for LS patients
- A dedicated clinical champion for hereditary CRC within every colorectal MDT
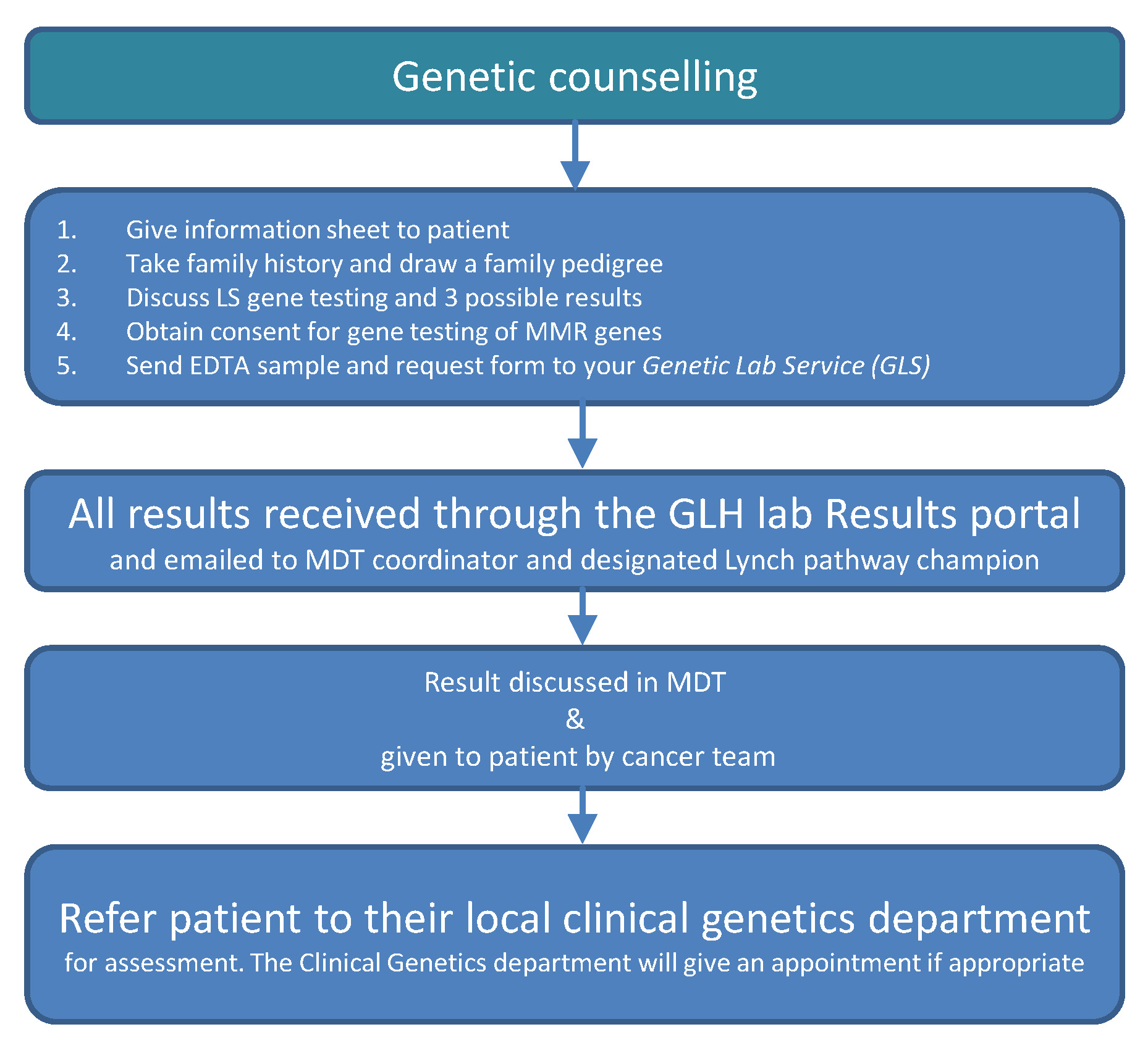
LS Testing pathway priorities
Priority 1: Identification of patients likely to have LS
- Colorectal MDTs nominate a ‘champion’ for this pathway
- Routine tumour testing for LS, performed preferentially on colonoscopic biopsies
- Subsequent reflex testing by pathologist for further somatic tests before germline testing, if required
- MDTs to identify and refer patients for genetic testing, either to their local clinical genetics service or their trained MDT clinicians
- QIP audit: to assess implementation of the QIP
Priority 2: Mainstreaming of germline genetic testing
- Online training modules for clinicians who wish to offer genetic testing
- Genetic testing offered in routine cancer appointments
- System to safely manage results and offer patients further advance specialised genetic services when applicable
- System for MDTs to refer patients and seek advice from regional genetics services
- Development of a regional LS registry
Team Members
Project lead:
Dr Kevin Monahan, Consultant Gastroenterologist, St Mark’s Hospital Lynch Syndrome & Family Cancer Clinic
Project managers:
Timothy Bill, Programme Lead, RM Partners
Laura Monje-Garcia, Nurse Practitioner, St Mark’s Hospital Centre for Familial Intestinal Cancer
CRC MDT pathway partners (governance through the RM Partners colorectal pathway group (PWG):
- London North West University Healthcare NHS Trust
- Chelsea and Westminster Hospital NHS Foundation Trust
- Imperial College Healthcare NHS Trust
- The Hillingdon Hospitals NHS Foundation Trust
- St George’s University Hospitals NHS Foundation Trust
- Kingston Hospital NHS Foundation Trust
- Croydon Health Services NHS Trust
- Epsom & St Helier University Hospitals NHS Trust
Regional Expert Centres:
- St Mark’s Hospital Lynch Syndrome & Family Cancer Clinic
- Northwick Park Hospital clinical genetics department (North West Thames)
- St George’s Hospital clinical genetics department (South West Thames)
- The Royal Marsden Hospital cancer genetics unit
If you would like to find out more please email LNWH-tr.SMFCC@nhs.net
Further information
Research article: Colorectal Disease Vol 25; Issue 7; July 2023
References
NICE DG27 guidance ‘Molecular testing strategies for Lynch syndrome in people with colorectal cancer’
NICE ‘Resource impact report: Molecular strategies for Lynch Syndrome in people with colorectal cancer’
Bowel Cancer UK campaign material ‘It’s time to test for Lynch Syndrome’